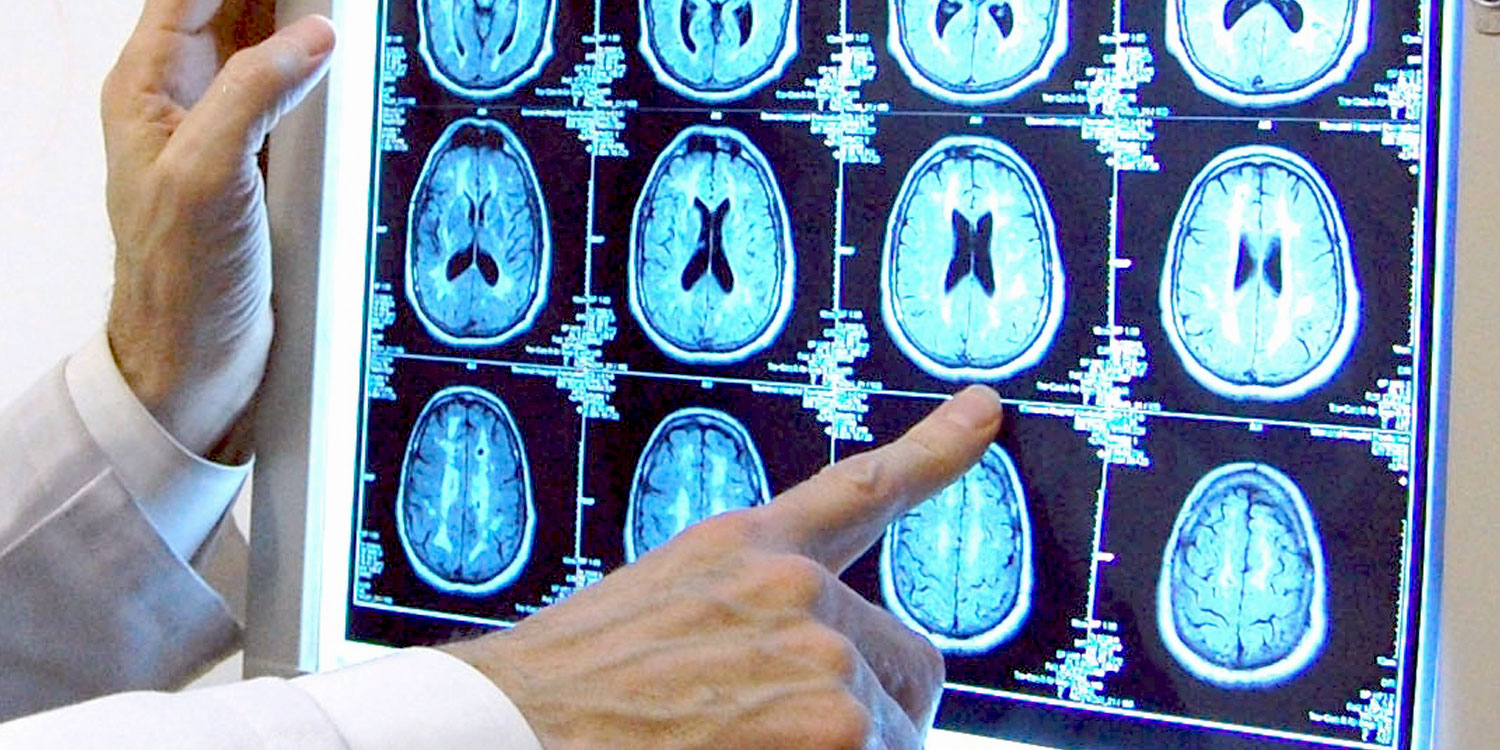
Affiliated with The Warren Alpert Medical School of Brown University, Butler Hospital’s clinical research trials provide valuable information on brain-based diseases. Through our research, new treatments are being discovered for diseases including depression and anxiety, obsessive-compulsive disorder (OCD), Alzheimer’s disease, Movement Disorders, such as Parkinson’s disease, and addictions.
Butler Hospital
345 Blackstone Blvd.
Providence, RI 02906
Learn more about Butler Hospital's research relating to mood disorders, obsessive-compulsive disorders, memory disorders, movement disorders as well as other studies researching aspects of mental health.
To view our current studies, click here.

An affiliate of The Warren Alpert Medical School of Brown University, MAP has a 20+ year history of excellence in clinical care, training, and cutting-edge research aimed at developing new and better ways to detect, treat, and someday even prevent Alzheimer’s.
Copyright © 2023 Care New England Health System