 Butler Hospital Administers World’s First Treatment of New Alzheimer's Drug, Aduhelm"
class="bg-img"
fetchpriority="high"
loading="eager"
decoding="async">
Butler Hospital Administers World’s First Treatment of New Alzheimer's Drug, Aduhelm"
class="bg-img"
fetchpriority="high"
loading="eager"
decoding="async">
News from the Memory and Aging Program at Butler Hospital
June 16, 2021
The world’s first infusion of Aduhelm to be given outside of a clinical trial was administered on Wednesday, June 16, at Butler Hospital in Providence, Rhode Island. It is the first drug approved to lower amyloid plaques to help slow the progression of Alzheimer’s disease (AD) in people diagnosed with mild cognitive impairment (MCI) or early AD. The U.S. Food and Drug Administration (FDA) granted accelerated approval for Aduhelm on June 7. The drug was developed by Massachusetts-based pharmaceutical company Biogen and Tokyo, Japan-based Eisai Co., Ltd.
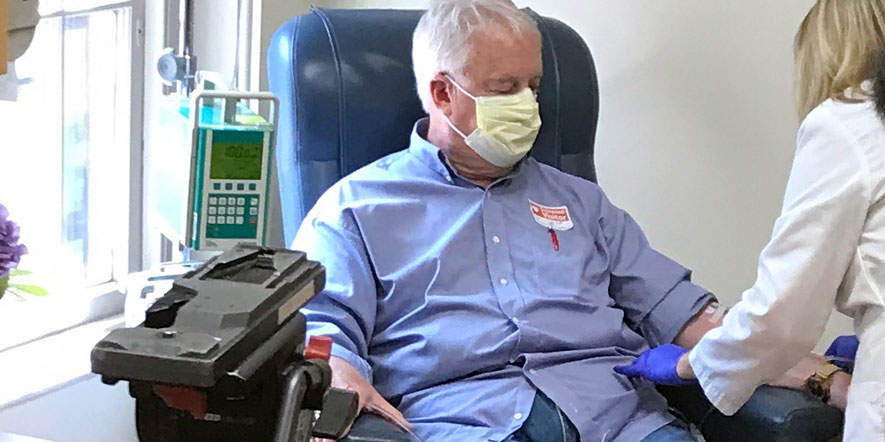
Marc Archambault of South Kingstown, RI, a research participant at the Memory and Aging Program at Butler Hospital diagnosed with early-onset Alzheimer’s Disease, was the recipient of the first Aduhelm treatment.
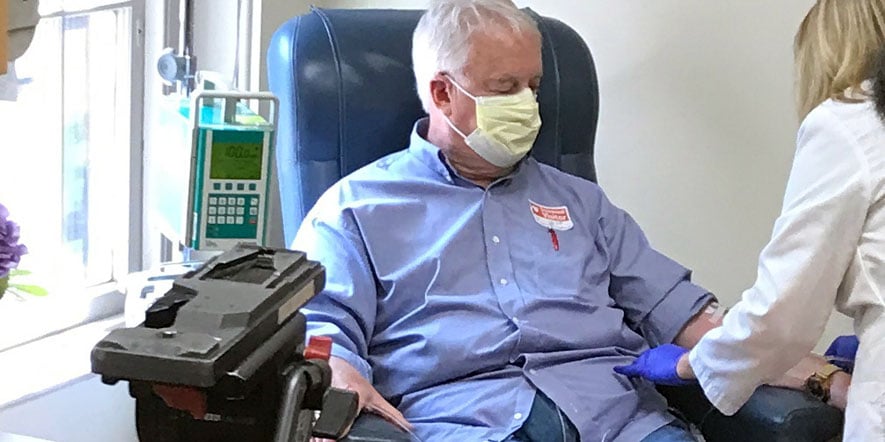
“I am a happy guy but hearing that the FDA had approved Aduhelm and that I am eligible for the treatment, I am living happier of course,” Archambault said. “The thought that the last stage [of Alzheimer’s] may now be far away for me, or even that I might stay as I am, is incredible. I feel very lucky to have the opportunity to receive this treatment at Butler Hospital with Dr. Stephen Salloway and his great team. People who have Alzheimer’s and their family, especially caregivers, know how hard this disease is. Hope and empathy needs to be in action for us, and for all people who have a disease with few treatments available.”
Alzheimer’s disease afflicts some 5 million Americans and 50 million people around the world. The FDA approved Aduhelm under its accelerated approval program, which is used for emerging treatments aimed at serious diseases for which there are currently few or no treatments. As part of the accelerated approval process, Biogen will conduct a Phase 4 post-approval clinical trial to verify the drug’s benefit. During that time it will be available to eligible patients like Archambault at sites across the nation.
Rhode Island contributed one of the largest number of participants enrolled in the clinical studies that led to Aduhelm’s approval, through study sites at Butler Hospital and Rhode Island Hospital, both of which are affiliates of the Warren Alpert Medical School of Brown University.
Stephen Salloway, MD, MS, director of Neurology and the Memory and Aging Program at Butler Hospital, the Martin M. Zucker professor of Psychiatry and Human Behavior and professor of Neurology at the Warren Alpert Medical School of Brown University, and Associate Director of Brown University’s Center for Alzheimer’s Research, served as co-chair of the global investigator steering committee for Aduhelm’s Phase 3 studies.
“Today we open a new era in the treatment of Alzheimer’s disease,” Dr. Salloway said. "We will now diagnose AD earlier with more specific tests and with a treatment aimed at slowing its progression. The real heroes are the thousands of study participants who put themselves on the line to make this advance possible. This is a turning point offering new hope for patients and families and this is only the beginning as we move forward to develop blood tests and treatments."
The clinical trials that led to aducanumab’s FDA approval included two Phase 3 trials, EMERGE and ENGAGE, in patients with early-stage Alzheimer’s disease. Significant amyloid lowering was seen in both studies. In EMERGE, patients who received aducanumab experienced significant slowing of decline on measures of cognition and function such as memory, orientation and language. Patients also experienced slowing of decline on activities of daily living including conducting personal finances, performing household chores, such as cleaning, shopping and doing laundry, and independently traveling out of the home. ENGAGE showed no drug-placebo difference in the primary outcome.
The aducanumab clinical program also included the Phase 1b PRIME study and its long-term extension (LTE) in patients with early Alzheimer’s disease. The results of this study indicated that aducanumab reduced the amyloid beta plaque in the brain that is associated with the development of AD. The drug was shown to reduce the presence of plaques in the brain as well as a reduction in the progression of symptoms a in a dose- and time-dependent fashion.
Eligibility criteria and other information for those interested in seeking treatment with Aduhelm at Butler Hospital is expected to be available in the coming days, on the Memory and Aging Program website at butler.org/memory or by calling (401) 455-6402 or e-mailing memory@butler.org.
Disclaimer: The content in this blog is for informational and educational purposes only and should not serve as medical advice, consultation, or diagnosis. If you have a medical concern, please consult your healthcare provider or seek immediate medical treatment.
Copyright © 2026 Care New England Health System