 Butler Hospital among first in U.S. to launch new study of promising Alzheimer’s treatment"
class="bg-img"
fetchpriority="high"
loading="eager"
decoding="async">
Butler Hospital among first in U.S. to launch new study of promising Alzheimer’s treatment"
class="bg-img"
fetchpriority="high"
loading="eager"
decoding="async">
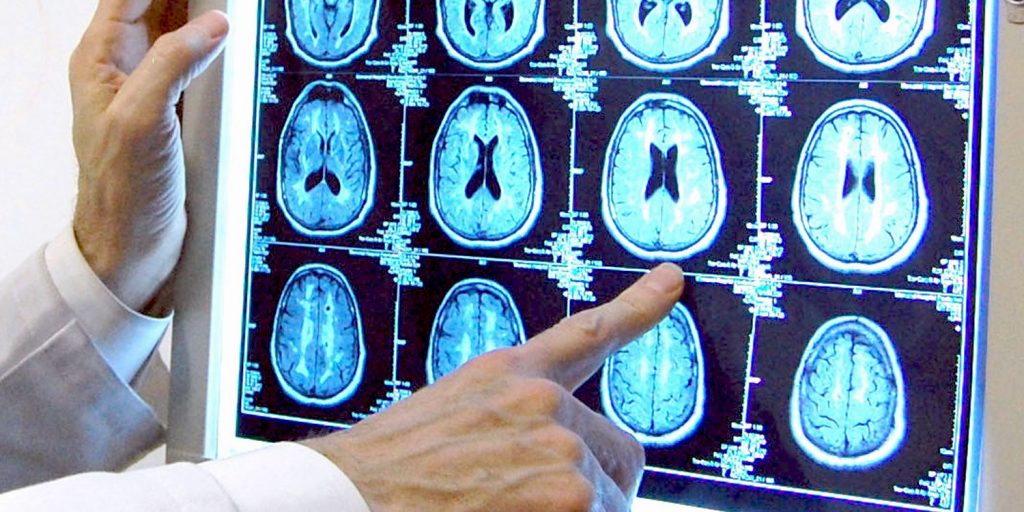 Dr. Stephen Salloway of the Memory and Aging Program at Butler Hospital points at brain scan images that show the beta amyloid plaques in the brain associated with the development of Alzheimer's disease.
Dr. Stephen Salloway of the Memory and Aging Program at Butler Hospital points at brain scan images that show the beta amyloid plaques in the brain associated with the development of Alzheimer's disease.
AHEAD 3-45 is a Phase III, international, multicenter clinical trial with 100 study sites in the US, Japan, Canada, Australia, Singapore, and Europe. It will study the effectiveness of BAN-2401, an investigational drug that selectively binds to, neutralizes and eliminates the amyloid beta proteins in the brain that are thought to be a causative factor for AD.
It is a double-blind, randomized study open to individuals ages 55 to 80 who are cognitively normal but have either elevated or intermediate levels of amyloid beta protein in the brain. A total of 1400 participants will be enrolled and treated with BAN2401 for 216 weeks.
AHEAD 3-45 is the first study conducted by the Alzheimer's Clinical Trials Consortium (ACTC), a critical new public-private partnership to fight AD. ACTC is a clinical trial network focused on accelerating and expanding studies of therapies for AD and related dementias. It is funded by the National Institute on Aging, part of the National Institutes of Health, and has 35 primary clinical study sites across the U.S., including Butler Hospital which is a founding member.
ACTC is partnering with Eisai Co., Ltd., a leading global research and development-based pharmaceutical company, to conduct the study which will be led by academic principal investigators from three ACTC member sites: Dr. Paul Aisen from the University of Southern California, and Drs. Reisa Sperling and Keith Johnson from Brigham and Women’s Hospital and Massachusetts General Hospital, Harvard Medical School, in partnership with Eisai.
“The AHEAD 3-45 study is breaking new ground by testing a drug to remove amyloid plaques much earlier to prevent and delay memory loss,” said Stephen Salloway, MD, MS, director of neurology and the Memory and Aging Program at Butler Hospital, the Martin M. Zucker professor of Psychiatry and Human Behavior and professor of neurology at the Warren Alpert Medical School of Brown University, and a member of the ACTC Executive Committee.
“This is a particularly innovative study because it focuses on stopping the cognitive impairment caused by Alzheimer’s even before it begins, and because the study is open to individuals as young as 55 who have low to moderate levels of amyloid beta protein in the brain but not yet any symptoms of Alzheimer’s,” Dr. Salloway said. “As a founding member of ACTC, we at the Butler Hospital Memory and Aging Program are excited to join with other Alzheimer’s experts across the nation and locally to accelerate the development of effective treatments for Alzheimer’s disease, and we’re excited about the potential impact that the AHEAD 3-45 study may have on the future of Alzheimer’s disease.”
After a common screening period in AHEAD 3-45, participants will be enrolled into one of two randomized, double-blind, placebo-controlled trials based on the level of amyloid in the brain: the A45 trial and the A3 trial. The A45 trial will enroll cognitively unimpaired participants who have elevated levels of amyloid in the brain, and aims to prevent cognitive decline and suppress the progression of brain AD pathology with BAN2401 administration. The A3 trial will enroll cognitively unimpaired participants who have an intermediate amount of amyloid in the brain, and who are at high risk for further amyloid beta accumulation.
Currently, BAN2401 is being studied in another pivotal Phase III clinical study called Clarity AD, also being conducted at Butler Hospital. The Clarity AD study is testing the effectiveness of BAN2401 in treating people who already have early Alzheimer’s Disease. It is open to individuals ages 50-90 years old with a diagnosis of Mild Cognitive Impairment or mild Alzheimer’s disease.
Those interested in participating in the AHEAD 3-45 study or the Clarity AD study are encouraged to join the Butler Hospital Alzheimer’s Prevention Registry online at butler.org/ALZregistry. The registry is used to screen interested individuals to see if they are likely to qualify for these and other studies being conducted at Butler Hospital focused on Alzheimer’s prevention or treatment. Interested individuals may also call the program at (401) 455-6402 or e-mail memory@butler.org.
Disclaimer: The content in this blog is for informational and educational purposes only and should not serve as medical advice, consultation, or diagnosis. If you have a medical concern, please consult your healthcare provider or seek immediate medical treatment.
Copyright © 2026 Care New England Health System