 Drug studied at Butler Hospital could become first to meaningfully change course of Alzheimer’s"
class="bg-img"
fetchpriority="high"
loading="eager"
decoding="async">
Drug studied at Butler Hospital could become first to meaningfully change course of Alzheimer’s"
class="bg-img"
fetchpriority="high"
loading="eager"
decoding="async">
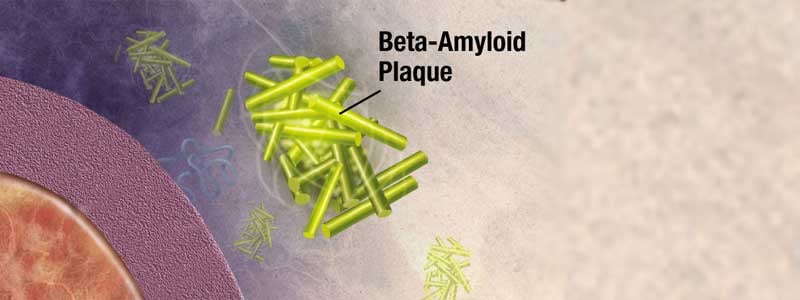
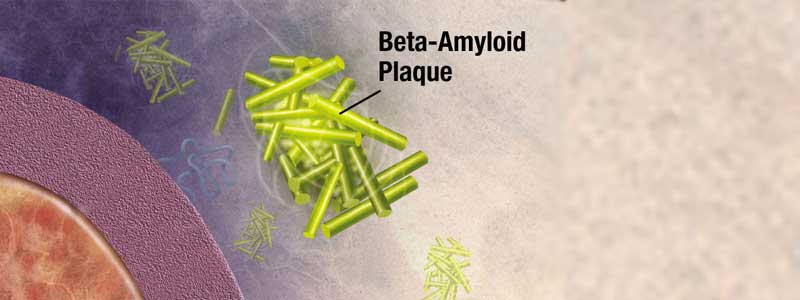
It was announced on July 8 that aducanumab, an investigational drug for the treatment for Alzheimer’s disease, has been submitted to the U.S. Food and Drug Administration (FDA) for approval with a request for Priority Review. Rhode Island contributed to the largest number of participants enrolled in the studies that led to the submission for approval, through study sites at the Memory and Aging Program at Butler Hospital and the Alzheimer’s Disease and Memory Disorders Center at Rhode Island Hospital, both of which are affiliates of the Warren Alpert Medical School of Brown University. If approved, aducanumab would become the first therapy to reduce the clinical decline of Alzheimer’s disease and would also be the first therapy to demonstrate that removing amyloid beta from the brain resulted in better clinical outcomes.
The drug’s makers, Biogen and Eisai Co., Ltd. (Tokyo, Japan), completed the submission of the Biologics License Application (BLA) to the FDA. The submission includes clinical data from the Phase 3 EMERGE and ENGAGE studies, as well as the Phase 1b PRIME study. Stephen Salloway, MD, MS, director of neurology and the Memory and Aging Program at Butler Hospital and the Martin M. Zucker professor of Psychiatry and Human Behavior and professor of neurology at the Warren Alpert Medical School of Brown University served as co-chair of the global investigator steering committee for the aducanumab Phase 3 studies.
“The submission of aducanumab for FDA approval represents a milestone in the fight against Alzheimer’s disease and we are excited that so many Rhode Islanders contributed to making this happen,” Dr. Salloway said. “For many people living with the early stages of Alzheimer’s disease, maintaining independence for as long as possible is the ultimate goal. If we can help slow the progression from one stage to the next, this could preserve independence, which, in turn, could have truly meaningful benefits for people living with the disease and their loved ones. Aducanumab represents a potential breakthrough that we hope will provide a treatment foothold in the fight against Alzheimer’s disease.”
"Two clinical studies, ENGAGE and EMERGE, suggested that not only did aducanumab reduce amyloid plaques in patients with Alzheimer’s disease but it also provided a meaningful reduction in worsening of clinical symptoms. The results of the EMERGE trial provide evidence that clinical trials in the disease can succeed, and that this breakthrough is a significant step toward conquering the disease over the long run. If approved by the FDA, aducanumab would be the long-awaited first therapy to slow the progression of Alzheimer’s disease, and a significant achievement given that no new medication has been approved in the Alzheimer’s disease field since 2003," said Dr. Jonathan Drake, associate director of the Alzheimer’s disease and Memory Disorders Center at Rhode Island Hospital.
Michel Vounatsos, Chief Executive Officer at Biogen said, “Alzheimer’s disease remains one of the greatest public health challenges of our time. It robs memories, independence and eventually the ability to perform basic tasks from the people we love. The aducanumab BLA is the first filing for FDA approval of a treatment that addresses the clinical decline associated with this devastating condition, as well as the pathology of the disease. We are committed to driving progress for the Alzheimer’s disease community and look forward to the FDA’s review of our filing.”
“People living with Alzheimer’s, their families, caregivers and so many others in the community are fighting this disease every day, and the global social burden of the disease is expected to grow as the population ages,” said Dr. Haruo Naito, Chief Executive Officer at Eisai Co., Ltd. “The BLA submission is an important step in the fight against this disease, for which pathophysiological progression currently cannot be stopped, delayed or prevented.”
The aducanumab clinical development program included two Phase 3 trials, EMERGE and ENGAGE, in patients with early stage Alzheimer’s disease (enrolled patients had mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease dementia with Mini-Mental State Examination (MMSE) scores of 24-30). In EMERGE, patients who received aducanumab experienced significant slowing of decline on measures of cognition and function such as memory, orientation and language. Patients also experienced slowing of decline on activities of daily living including conducting personal finances, performing household chores, such as cleaning, shopping and doing laundry, and independently traveling out of the home.
EMERGE (n=1,638) met its pre-specified primary endpoint, with patients treated with high dose aducanumab showing a statistically significant reduction of clinical decline from baseline in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scores at 78 weeks (22% versus placebo, P=0.01). In EMERGE, patients treated with high dose aducanumab also showed a consistent reduction of clinical decline as measured by the pre-specified secondary endpoints: the Mini-Mental State Examination (MMSE; 18% versus placebo, P=0.05), the Alzheimer’s Disease Assessment Scale-Cognitive Subscale 13 Items (ADAS-Cog 13; 27% versus placebo, P=0.01) and the Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory Mild Cognitive Impairment Version (ADCS-ADL-MCI; 40% versus placebo, P=0.001). Imaging of amyloid plaque deposition in EMERGE demonstrated that amyloid plaque burden was reduced with low and high dose aducanumab compared to placebo at 26 and 78 weeks (P<0.001). While ENGAGE (n=1,647) did not meet its primary endpoint, Biogen believes a subset of data from ENGAGE are supportive of the outcome in EMERGE.
The aducanumab clinical program also included the Phase 1b PRIME study and its long-term extension (LTE) in patients with early Alzheimer’s disease (enrolled patients had prodromal Alzheimer’s disease or mild Alzheimer’s disease dementia with MMSE scores of 20-30). The results of this study indicated that aducanumab reduced amyloid beta plaque in a dose- and time-dependent fashion, and analyses of exploratory clinical endpoints showed a reduction of clinical decline (CDR-SB and MMSE, nominally statistically significant for the 10 mg/kg dose at 12 months), which continued out to 48 months in the LTE.
The completion of the BLA submission followed a planned pre-BLA meeting with the FDA. The FDA now has up to 60 days to decide whether to accept the application for review, at which point, if accepted, Biogen expects the FDA will also inform the Company whether the BLA has been granted Priority Review designation. The BLA will then be subject to review by the FDA to make a determination on the potential approval of aducanumab.
In addition to submitting the BLA to the FDA, Biogen has continued to engage in dialogue with regulatory authorities in other markets, including Europe and Japan, working diligently toward the goal of submitting applications in these markets.
About the Memory and Aging Program at Butler Hospital
The Memory & Aging Program (MAP) at Butler Hospital is a worldwide leader in Alzheimer’s disease research and a local Rhode Island partner in the fight against Alzheimer’s and other forms of dementia. An affiliate of The Warren Alpert Medical School of Brown University, MAP has a 25-year history of excellence in Alzheimer’s clinical care, training, and research aimed at developing new and better ways to detect, treat, and someday even prevent Alzheimer’s. Individuals who wish to be considered for participation in current and future research studies and clinical trials conducted at the Memory and Aging Program for the prevention and treatment of Alzheimer’s disease can join the program’s Alzheimer’s Prevention Registry at Butler Hospital online at butler.org/ALZregistry or by calling (401) 455-6402. For more information visit butler.org/memory and follow on Facebook and Twitter.
Related Resources
Alzheimer's Prevention Registry at Butler Hospital
Learn more about research studies at the Memory and Aging Program at Butler Hospital
If you're 40+ with normal memory or mild memory loss, you can help in the fight against Alzheimer's. Here's how: butler.org/ALZregistry
Disclaimer: The content in this blog is for informational and educational purposes only and should not serve as medical advice, consultation, or diagnosis. If you have a medical concern, please consult your healthcare provider or seek immediate medical treatment.
Copyright © 2026 Care New England Health System