 A second drug studied at Butler Hospital shows significant results in slowing Alzheimer's symptoms"
class="bg-img"
fetchpriority="high"
loading="eager"
decoding="async">
A second drug studied at Butler Hospital shows significant results in slowing Alzheimer's symptoms"
class="bg-img"
fetchpriority="high"
loading="eager"
decoding="async">
March 15, 2021
Providence, RI – The nation’s smallest state continues to play a large role in the development of promising potential treatments for Alzheimer’s disease (AD). Clinical trial results announced on Saturday and published in the New England Journal of Medicine (NEJM) indicate that the investigational drug donanemab holds promise as a potential treatment for early AD. The announcement is the latest in a series of promising steps forward in Alzheimer’s research with significant ties to Rhode Island.
The announcement of these study results follows the submission last summer of another investigational drug for the treatment of AD, aducanumab, for review by the FDA. Both drugs were studied in part at the Memory and Aging Program at Butler Hospital and at the Alzheimer’s Disease and Memory Disorders Center at Rhode Island Hospital, both of which are affiliates of the Warren Alpert Medical School of Brown University.
Stephen Salloway, MD, MS, director of the Memory and Aging Program and of Neurology at Butler Hospital and the Martin M. Zucker professor of Psychiatry and Human Behavior and professor of Neurology at the Warren Alpert Medical School of Brown University, is a co-author of the NEJM article. He was principal investigator for the TRAILBLAZER study at Butler Hospital and was a lead investigator on the trial.
“This is yet another significant and encouraging milestone in what has proven to be a momentous year in the fight against Alzheimer’s disease. In the last twelve months we’ve seen significant advancements in diagnosing and treating Alzheimer’s,” Dr. Salloway said. "With this kind of momentum we are on the verge of important breakthroughs for Alzheimer's disease. The Memory and Aging Program and our partners and colleagues in research across Rhode Island have played a large part in those advancements, as have the many Rhode Islanders who have selflessly participated in these studies. I’m just so grateful for that collaboration and participation. It’s what is fueling every step forward that we collectively take in this fight.”
"With this kind of momentum we are on the verge of important breakthroughs for Alzheimer’s disease." - Dr. Stephen Salloway
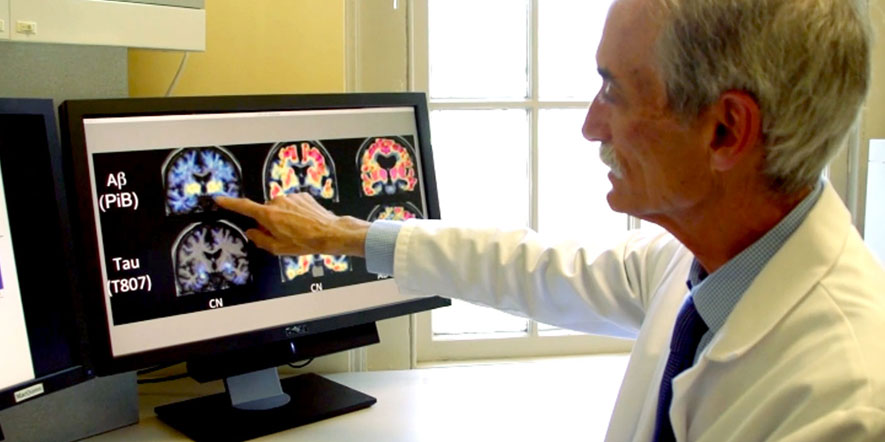
Eli Lilly and Company, maker of donanemab, announced the study’s findings on Saturday at the International Conference on Alzheimer’s & Parkinson Disease 2021 (AD/PD™21), in tandem with the publication of the article in the NEJM. Its phase 2 study of donanemab, called TRAILBLAZER-ALZ, showed that the drug resulted in significant slowing of decline in a composite measure of cognition and daily function in people with early symptomatic AD compared to placebo. The drug works by targeting the amyloid plaque and tau protein build-up in the brain that is associated with the development of Alzheimer’s disease and other forms of dementia.
“With more than 30 years of dedication to finding solutions for this devastating disease, Lilly has set out to change how we diagnose and treat Alzheimer’s. We’ve created a set of tools that allows us to see and measure brain pathology directly and this has unlocked new ways to conduct trials which we believe is the path forward for continued progress in AD,” said Daniel Skovronsky, M.D., Ph.D., Lilly’s chief scientific officer and president of Lilly Research Laboratories. “We are grateful to the patients, caregivers and investigators who participated in the TRAILBLAZER-ALZ study and we aim to replicate these finds for donanemab in a future study.”
The TRAILBLAZER-ALZ study was conducted at 61 research sites across the U.S. and Canada, including the two sites in Rhode Island. It utilized new imaging technology, tau Positron Emission Tomograhy (PET) imaging with flortaucipir tracer, that was developed specifically for the detection of tau protein in the brain. These tracers were developed in part at the Memory and Aging Program at Butler Hospital in partnership with the Alzheimer’s Disease and Memory Disorders Center at Rhode Island Hospital. Dr. Salloway was a lead study clinician through all phases of the development of the flortaucipir tracer.
“The immediate goal is to provide treatments that will slow cognitive impairment in people experiencing the early stages of Alzheimer’s disease. At the same time we’re also testing treatments to prevent or delay memory loss in people at risk. We are on the cusp of a watershed moment in Alzheimer’s disease treatment that could change the lives of millions of people around the world.” Dr. Salloway said.
There are currently over 50 million people living with dementia around the world, with numbers expected to increase to nearly 152 million by 20502. Almost 10 million new cases of dementia are diagnosed each year worldwide, with one new case every 3 seconds and a significant increase in the caregiving burden placed on society and families. In the US alone, there was an increase of 8 million new caregivers from 2015 to 20203. The current annual societal and economic cost of dementia is estimated at $1 trillion, an amount that is expected to double by 2030 unless effective means for early diagnosis and treatment of the disease are found.
About the Memory and Aging Program at Butler Hospital
The Memory & Aging Program (MAP) at Butler Hospital is a worldwide leader in Alzheimer’s disease research and a local Rhode Island partner in the fight against Alzheimer’s and other forms of dementia. An affiliate of The Warren Alpert Medical School of Brown University, MAP has a 25-year history of excellence in Alzheimer’s clinical care, training, and research aimed at developing new and better ways to detect, treat, and someday even prevent Alzheimer’s. Individuals who wish to be considered for participation in current and future research studies and clinical trials conducted at the Memory and Aging Program for the prevention and treatment of Alzheimer’s disease can join the program’s Alzheimer’s Prevention Registry at Butler Hospital online at butler.org/ALZregistry or by calling (401) 455-6402. For more information visit butler.org/memory and follow on Facebook and Twitter.
Disclaimer: The content in this blog is for informational and educational purposes only and should not serve as medical advice, consultation, or diagnosis. If you have a medical concern, please consult your healthcare provider or seek immediate medical treatment.
Copyright © 2026 Care New England Health System